Increasing Access for Rare Disease Patients Globally
Patients who suffer from rare diseases often lack access to innovative treatment options because the manufacturer of the medicine cannot justify the investment in registering and commercializing the medicine in countries where patient populations are low. Managed Access Programs provide manufacturers of innovative medicines for rare diseases a cost-effective option to provide patients access in countries where the medicine is not commercially available. If a physician chooses to prescribe such a medicine, there are regulatory pathways for obtaining access. Tanner Pharma works with pharmaceutical manufacturers and physicians to bridge the gap in access to innovative medicines for rare diseases.
What are Tanner Access Solutions?
“Managed Access” can span many specific needs. It often covers many types of programs, in particular “Compassionate Use”, “Named Patient Supply”, “Expanded Access”, “Early Access”, “Donation Programs”, “(ATU)” and others.
Tanner Access Solutions spans all of these. We are a full-service provider for Expanded Access Programs (EAPs) in the United States (US), Drug Shortages, Global Access, Post-Trial Supply, Donation Programs, Compassionate Use Programs, Market Withdrawal, Compassionate Use, and Advisory Services.
EAPs provide a mechanism from the Food and Drug Administration (FDA) for patients living in the US to get early access to a medicine in development when they have run out of treatment options. Under strict conditions, products in development can be made available to individuals or groups of patients who have a disease with no satisfactory authorized therapies and who do not meet the inclusion criteria to enter a clinical trial or cannot gain access to a trial site. Requests are made by the physician or the biopharmaceutical company to the FDA.
“Compassionate Use” (“CU”) is a mechanism outside the US that allows the use of an unlicensed medicine prior to approval anywhere in the world. Under strict conditions, products in development can be made available to individuals or groups of patients who have a disease with no satisfactory authorized therapies and who cannot enter clinical trials. Requests are made by the physician to their local competent authority in the relevant country.
“Named-patient program or named patient supply” is another way to provide patients and physicians outside the US access to medicines that are approved somewhere in the world, but not available to them in their own country. Similarly to CU, the physician must make request to their local competent authority to treat an individual patient under their direct responsibility.
In all situations the request must be unsolicited, and the following conditions must be fulfilled:
- The patient/s suffer from a life-threatening, long-lasting or seriously debilitating illness
- There are no currently authorized therapies available to treat the disease or condition in a satisfactory way
- The patient cannot enroll in an ongoing clinical trial
- The medicine/ therapy must be undergoing clinical trials or have entered the marketing-authorization application process
IMPACTING PATIENTS GLOBALLY
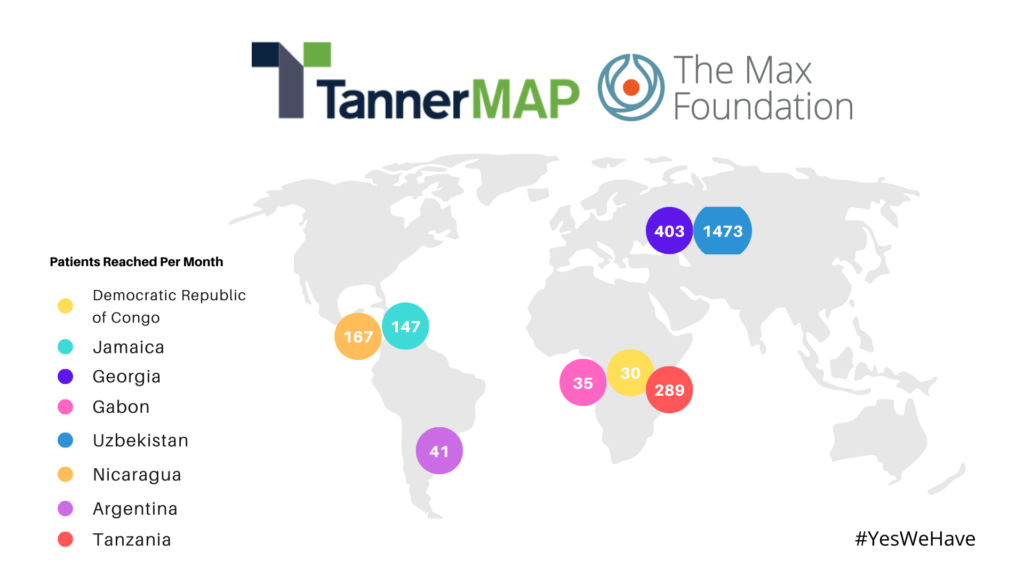
Beyond physicians or manufacturers, we also work with nonprofits. For example, we work with one of our partners, The Max Foundation to make eight (8) medicines on a donation basis for over 30,000 patients with chronic myeloid leukemia in nearly 80 low- and middle-income countries. Tanner has continued to develop capabilities for these Donation Programs that biopharmaceutical companies see as a humanitarian pathway aligned to their commitment to corporate social responsibility. Tanner is also a member of Global Gene’s Corporate Alliance, a coalition of more than 80 rare disease industry stakeholders that are committed to collaboration between industry and patient communities to improve and expedite access to effective therapies for all rare disease patients.
Tanner’s approach to these programs is unique because of the operating model we employ giving physicians and their patients a much more personalized access experience. Tanner has a track record of finding solutions, even in very difficult scenarios, to get medicine to patients no matter their location and based on the specific needs.
Tanner is a trusted partner helping biopharmaceutical companies change the world one patient a time.
WHY PARTNER WITH TANNER FOR YOUR ACCESS PROGRAM
- Expand access to more markets and earlier than planned by utilizing Tanner’s global GDP-compliant supply chain and regulatory expertise to import medicines into countries where marketing is not yet approved
- Reduce the administrative workload by authorizing Tanner to manage the requirements of validating patients and institutions that participate in an access program
- Gain broader insights on product efficacy and demand to help inform future commercialization strategies and complement clinical trial findings by tapping into Tanner’s data collection and reporting services
- Build awareness with physicians, hospitals, pharmacists, regulators, and patients by letting Tanner help develop and deliver a customized and compliant access program