Enabling access continuity of medical treatments for patients
There are different instances where patients may experience interruptions in treatment access, entirely lose access they once had, or completely lack access to a commercialized medicine that just isn’t available where they live. The common denominator being that all factors are almost always beyond the patient’s control. Finding ways to mitigate and overcome these challenges in access is vital for patients’ long-term health and can make a life-saving difference.
Many factors can lead to a patient losing access to medication, including the end of their clinical trial, having products withdrawn from the market in certain countries, and supply chain disruptions. Additionally, there are instances where a treatment may not be commercialized in a patient’s home country for many years, if at all, furthering limiting patients.
For Tanner Pharma, ensuring access and access continuity is part of its overall mission to improve lives around the world. Tanner’s Access Programs, in addition to its supply chain distribution network, help to change the narrative and better enable patient access to medicines globally.
Managed access: beyond the commercial market
When a pharmaceutical or biotech company is registering its product, it will likely begin with a very limited commercial footprint. From launch, the path forward is not often straight and there are no guarantees that treatments will always reach the country where patients are in need. This can be detrimental to patients, especially those who suffer from rare diseases where treatment options are scarce to begin with.
“It may take years, if ever, in some countries, for medicine to be available to patients, and some patients just don’t have that time. So that’s where the MAP team steps in. We partner with manufacturers to set up Managed Access Programs, so called because we’re compliantly controlling or managing access of medicine that is not on the market in a specific country,” says John Lagus, EVP of Business Development at Tanner.
Most countries have pathways that allow patients to access unapproved medications when no other therapies are available on the market. “Early access programs,” “named patient supply” and “compassionate use” are some of the pathways that fall under this umbrella and offer opportunities to bring treatments to patients. But these programs can be difficult and time-consuming for manufacturers to navigate on a global scale and on their own.
“We have to consider the type of patients, and any other aspects of this program. Is there physician education that needs to happen? Are we capturing real-world data in these programs? There’s usually a lot of complexity that manufacturers would not know how to deal with and that’s where we can help.”
Managed access: post-trial continuity
An example of how Tanner may help manufacturers navigate the complexities of managed supply is in post-trial access (PTA). Post-trial access is a pathway that allows continuity of treatment for clinical trial participants until the product is available commercially or becomes locally accessible to them.
Globally, there is a lack of firm guidance when it comes to a manufacturer’s responsibility regarding treatment after a clinical trial has ended. However, it is a prevalent ethical issue, especially when it comes to rare diseases and oncology where treatment is typically expensive and may be in short supply.
While the Declaration of Helsinki, as well as CIOMS in collaboration with WHO, provide some guidance – particularly regarding disclosing information to clinical trial patients about PTA – there is still a lot of ambiguity that needs resolving.
Companies have an ethical responsibility to continue providing access for patients that were benefitting from a medicine on a clinical trial. Historically that has been done via open-label extension trials. Many companies are turning to managed access mechanisms of supply as a more cost-effective way to facilitate this access when the primary objective is maintaining treatment for these patients. And while patient wellbeing is the main priority of everyone involved, companies have to consider a country’s policy for unlicensed medicines for PTA. Tanner’s extensive network resources and expertise assist manufacturers in overcoming these challenges.
Meeting demand: market withdrawals and other immediate needs
Another way in which patients may lose access to medication is if a company withdraws its product from the market and the medication is no longer available in the patient’s country, or only available at a much higher price. A product can also get withdrawn from a market unknowingly in the event of a merger when the treatment may need re-registering with the new owner.
“One reason for a withdrawal program might be that a company has certain markets where a product is actually losing money,” adds Lagus. “Price reductions continue to drive down the price of medicines, so it doesn’t make sense for them to maintain the marketing authorizations in some territories. Rather than continue to sell it commercially, they will transition those patients to a managed or global access program.”
Drug shortages are another global issue that require immediate support and can affect patients in any country. The Covid-19 pandemic is one example where pharmaceutical and equipment availability was greatly impacted. However, drug shortages are hardly a new event and were very common pre-pandemic. Drug shortages can be caused by a number of factors, including supply chain disruption, manufacturing delays, shortage of an active ingredient, or just increase in demand and can happen across any therapeutic area.
The FDA’s report, Drug Shortages for Calendar Year 2021, highlights the number of new drug shortages from 2010 to 2021 as shown in Figure 1 below. They also note the number of ongoing shortages yet to be resolved from previous years in Figure 3. Great strides have been made over the years to mitigate these challenges, but shortages still arise in great frequency ultimately affecting the patient’s standard of life.
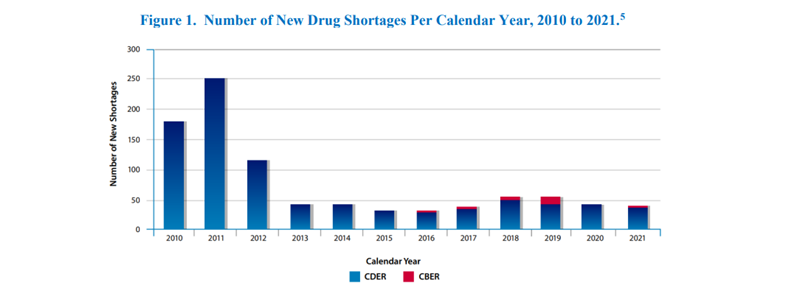
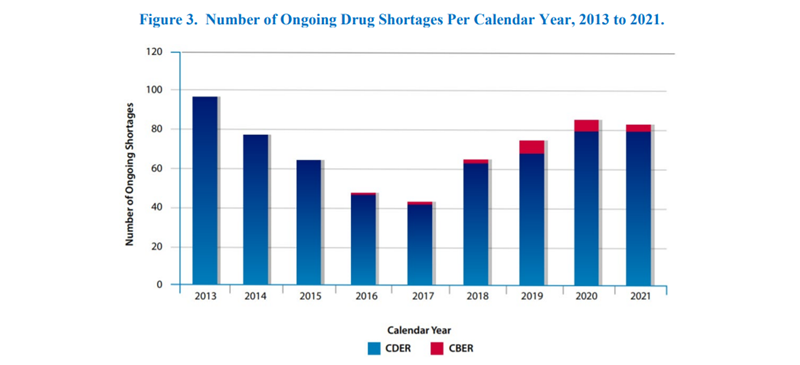
During a shortage, there may be a brief withdrawal period that only requires post-withdrawal supply for a short time. In other cases, when a treatment is permanently withdrawn, an immediate supply of a generic drug or alternative form of the withdrawn drug could be the solution.
Shortages rarely affect every country or region at the same time. And the likelihood is that the treatment will be available somewhere around the world. The important thing is knowing where to look for support when, or even before, these shortages arise. Global access programs can help to source the medicine in a country where it is available and supply it into a country where it is on shortage.
“In these cases, we can find access through a vetted supply chain, that in some cases is direct with the manufacturer to source the product immediately to supply the patient,” explains Maryori Alvarenga, EVP of TannerGAP.
“Our work is fast paced because we support an immediate demand. We have distributors, governments, NGOs, and hospitals coming to us with patient needs that require quick action, and we are actively fulfilling those requests.”
Maintaining access to medication amid supply chain disruption
The international pharma supply chain is highly complex, given its global reach and the sheer number of drug treatments subject to high demand. Though forecasting of demand is advanced, disruptions in the supply chain are frequent and there is a growing need to source treatments rapidly and compliantly.
Disruptions to the supply chain can have serious impacts on the availability of medication. A pharma supply chain must be robust and flexible to ensure that access is continuous even through unforeseen challenges. This was an event that far too many companies experienced at the height of Covid-19.
Due to the complexity of the manufacturing and distribution process for drug treatments, as well as the highly regulated nature of the industry, meeting patient needs and manufacturer expectations can be challenging. Tanner works with biotech and pharmaceutical companies, and a global partner network to ensure supply chain consistency. This includes temperature-controlled supply, handling global compliance, quality control, managing import/export clearance, and monitoring logistics.
Tanner Pharma Group is a US-based pharma services company that provides specialized access solutions around the world. Over more than two decades, Tanner has developed a portfolio of services driven by the determination to improve global access to medicines for patients through access programs, clinical trial solutions, and product licensing and commercialization in challenging markets.
Free Whitepaper
Download the whitepaper below to find out more about the services we provide and how Tanner can assist with access to vital medical treatments.
Maintaining Global Supply of Pharma Products Amid Uncertainty
Managing supplies of medical products is a continuous process that requires effective forward-planning in procurement combined with the flexibility to adapt to meet changing demands in a crisis. The peak of the global pandemic of 2020 really highlighted the life-changing importance of preparation and response, and what can happen when supplies become depleted. In this whitepaper, we examine some of the reasons behind the unavailability of medical products around the world and address various operations challenges and response protocols required to resolve these immediate demands, with expert commentary by our Tanner team. Download to learn more.
