Charlotte Company Stockpiling Drug in Europe to Treat Radiation and Chemical Exposure
The move was made due to Russia’s invasion of Ukraine and the escalating potential for incidents that would require the medication.
Originally posted on WCNC.com.
Author: Curtis Carden | Published: 9:25 PM EDT April 4, 2022 | Updated: 11:27 PM EDT April 4, 2022
CHARLOTTE, N.C. — An international distributor of medicines, based in Charlotte, is upping its inventory of a drug in Europe that helps treat radiation and chemical exposure.
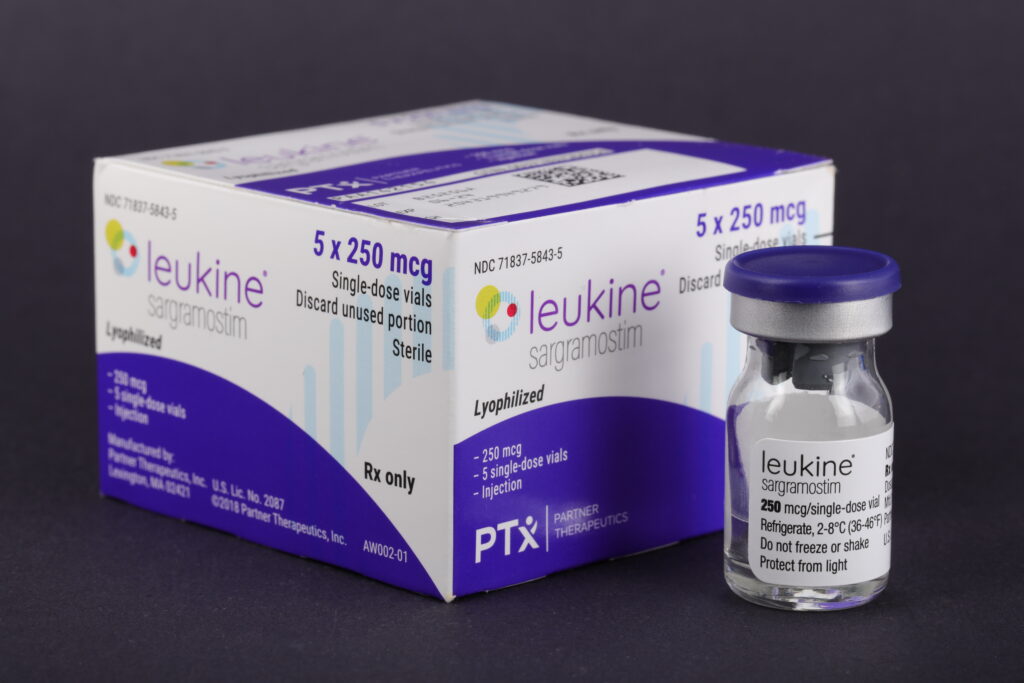
Tanner Pharma Group announced it is increasing the inventory of Leukine with the medication’s owner, Partner Therapeutics, in response to Russia’s invasion of Ukraine and the potential for incidents that could require a speedy deployment of the drug to treat people.
“In response to the ongoing conflict in Ukraine, Tanner is supporting preparedness and response in Europe by increasing the local inventory of Leukine that can be rapidly deployed in response to an emergency,” Banks Bourne, CEO and Founder of Tanner Pharma, said in a statement provided March 24. “The unique efficacy of Leukine, which has been shown to improve survival when given within 96 hours after radiation exposure and without whole blood transfusions, makes it a highly effective countermeasure with important logistical advantages in the event of a nuclear detonation. Positioning more supply in Europe ensures that more Leukine is available quickly, if needed.”
Leukine was approved by the FDA in 2018 as a way to treat acute radiation syndrome and has been held for use in the United States since 2013.
According to the FDA report, “Leukine was shown to increase survival when administered up to 48 hours after total body irradiation exposure at doses expected to be fatal to 50% of those exposed subjects under conditions of minimal supportive care.”
A press release provided by the company also mentioned that the drug was used to help treat some victims of the nuclear incident at the Chernobyl Nuclear Power Plant in 1986.
Steve Scalia, president of Tanner Pharma Group, spoke with WCNC Charlotte on April 4 and mentioned that at this time, Leukine does not have market authorization in Europe.
He said Tanner Pharma has the ability to use special access permits, much like an emergency use authorization (EUA), to be able to distribute the drug in Europe as long as it is closely monitored.
“We all try to do what we can,” Scalia explained. “We’re fortunate enough to have built a company that has its heart in the right place. We’re trying to make things better for people, especially in markets where medicine is not available. This Leukine example in Europe is only one example of the many programs we have, where we’re trying to bridge that gap between demand and supply, and provide access for people in need.”
Scalia said there as some impacts of Russia’s invasion that could spill out to not only Ukraine.
“We view this as a regional deployment,” he added. “Some of the inquiries out there are not just within the borders of Ukraine.”
Tanner Cares, a program offered by the company, takes a portion of proceeds gathered by the company that goes back into monetary and medicinal donations that can be provided to help areas. Scalia added the company is also working to help with the ongoing refugee crisis caused by the invasion.
Both Scalia and Bourne grew up in Charlotte. The president of the company said seeing the community’s togetherness in supporting Ukraine and others comes as no surprise.